IMAGE: Figures: Identification of a G protein-independent activator (GiGA1) of GIRK channels
view more
Credit: Paul Slesinger, PhD
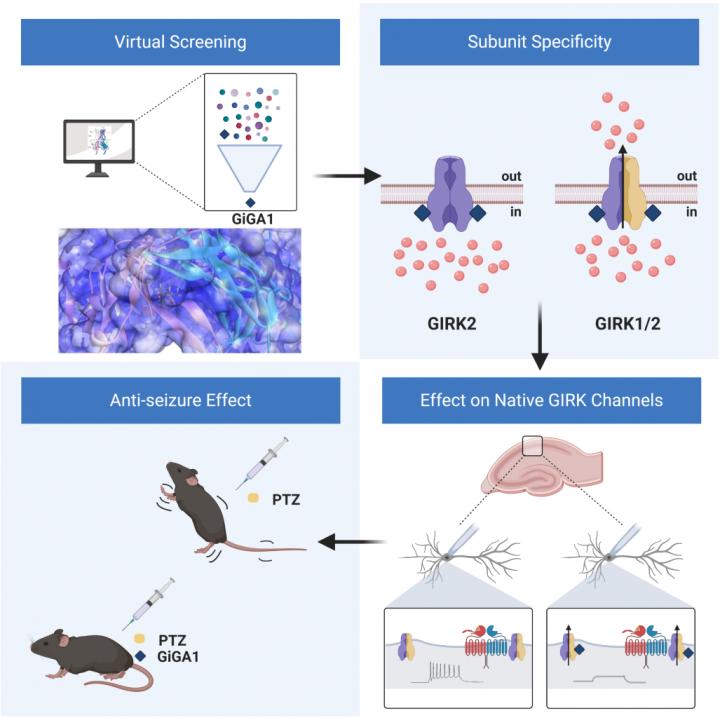
Bottom Line: Potassium channels play an important role in controlling the electrical activity of neurons in the brain. We identified and characterized a new compound, called GiGA1, that selectively opens a subclass of potassium channels and can functionally suppress seizures in an epilepsy animal model.
Results: GiGA1 was identified in a library of ~750 thousand chemical compounds, using computer-based modeling and virtual screening of an alcohol-binding pocket in the channel. GiGA1 preferentially activates a subset of GIRK channels, those containing the GIRK1 and GIRK2 subunits, most commonly found in the brain. GiGA1 also activates natively expressed GIRK channels in hippocampal neurons and in turn reduces neuronal excitability. Systemic administration of GiGA1 exhibits anti-seizure properties in an acute epilepsy animal model.
Why the Research Is Interesting: Two reasons. First, the study highlights an integrated approach of identifying ion channel modulators with subunit-specificity. Second, the newly discovered activator of GIRK channels could provide potential treatments for brain disorders, including epilepsy and alcohol use disorder.
Who: Activation of an ion channel in the brain, called GIRK channels, with a selective chemical compound reduces seizures in mice.
When: A systemic injection of GiGA1 15-30 min prior to inducing convulsions significantly reduced the severity of seizures.
What: The study identified and characterized GiGA1-induced GIRK activity and studied its effect on brain neurons and on suppressing seizures in an epilepsy animal model.
How: Utilizing a structural model of an alcohol bound GIRK channel for virtual screening and subsequent functional analysis, we identified a GIRK channel activator (GiGA1). Single-cell electrophysiology demonstrated that GiGA1 has similar properties as alcohol, but is more potent, and activates natively expressed GIRK channels in the hippocampus. Systemic administration of GiGA1 prior to inducing convulsions with pentylenetetrazole (PTZ) significantly reduces the number and severity of seizures.
Study Conclusions: We identified GiGA1 through a virtual screen with ~750 thousand small-molecule compounds and a structural model of the alcohol pocket in GIRK channels. GiGA1 selectively activated GIRK1-containing channels, and, like alcohol, was G protein-independent. GiGA1 activated endogenous GIRK channels in the brain, and suppressed drug-induced seizures.
Paper Title: Identification of a G protein-independent activator (GiGA1) of GIRK channels
Said Mount Sinai’s Dr. Paul Slesinger of the research:
This study ‘walks’ people through the process of identifying new compounds that selectively modify neuronal activity in the brain. We focused on a particular type of ion channel, called a GIRK channel, which provides a major source of inhibition in the brain. GIRK channels are implicated in a variety of neurological disorders, and are known to be directly affected by alcohol. We took advantage of our structural understanding of how alcohol modulates GIRK channels, and ultimately identified a subunit-specific compound that activates GIRK channels and mitigates convulsant activity. This study was a collaborative effort with the groups of Dr. Schlessinger at Mount Sinai and Dr. Marugan at the National Center for Advancing Translational Sciences at NIH.
###
Relevant Social Media handles:
Twitter: @SlesingerLab; @paslesin; @CNB_MountSinai; @SchlessingerLab; @ncats_nih_gov, @CellPressNews, @NIAAAnews
Facebook: https:/
Instagram: niaaanews,
LinkedIn: http://www.
https:/
To request a copy of the paper or to schedule an interview with Dr. Sleginger, please contact Mount Sinai’s Director of Media and Public Affairs, Elizabeth Dowling, at elizabeth.dowling@mountsinai.org or at 347-541-0212.
Corresponding Authors:
Paul A. Slesinger, PhD, Lillian and Henry M. Stratton Professor of Neuroscience, Director, Center for Neurotechnology & Behavior, Icahn School of Medicine at Mount Sinai, New York (lead)
Avner Schlessinger, PhD, Associate Professor, Department of Pharmacological Sciences, Associate Director, Mt. Sinai Center for Therapeutics Discovery and other coauthors.
Juan Jose Marugan, Ph.D., Group Leader, NIH Chemical Genomics Center, National Center for Advancing Translational Sciences, National Institutes of Health, Rockville, MD
TDnews














