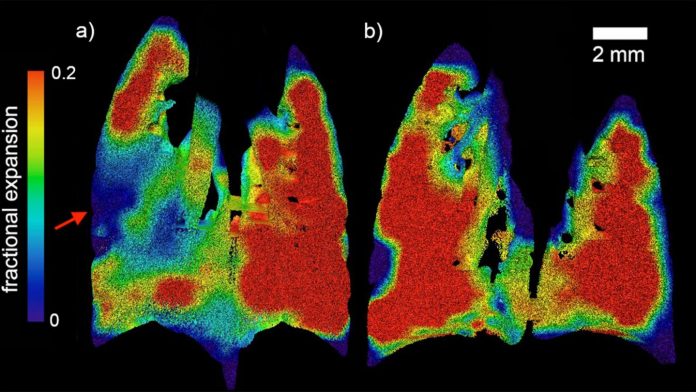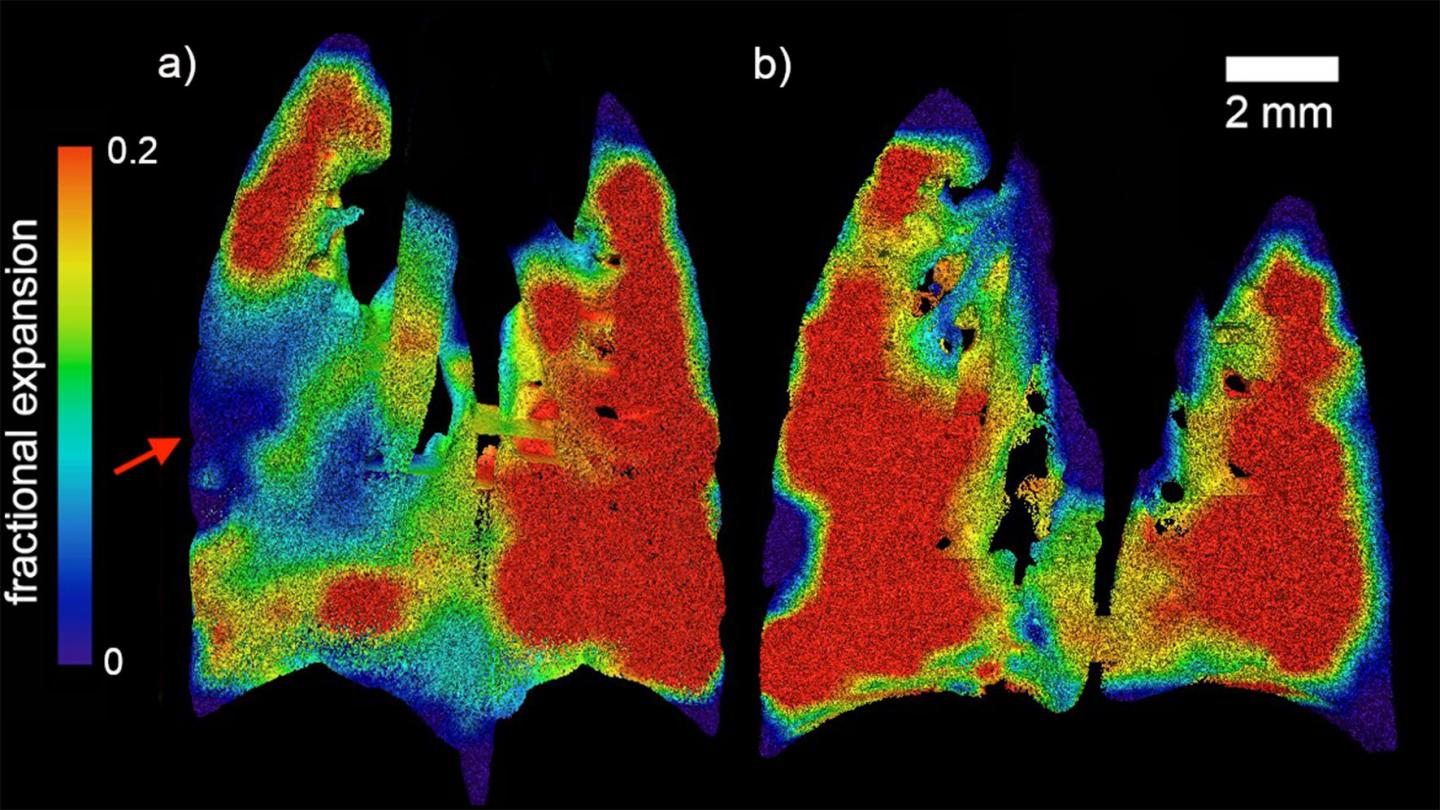
IMAGE: Regional fractional expansion of the lung since the beginning of the breath is characterised as a fraction of the XV voxel. Coronal slices of the expansion at peak inspiration are…
view more
Credit: Monash University
- – World-first research, led by Monash University, has developed radical non-invasive X-ray technology aimed at helping diagnose, treat and manage people with cystic fibrosis.
– The technology can pinpoint localised areas of deficiency in a lung, offering potential for faster and more accurate diagnoses.
– Researchers can track the movement of air through the lungs, improving their capacity to assess lung function in both healthy and diseased lungs.
World-first research led by Monash University could hold the key to better monitoring and treatment of lung disease associated with cystic fibrosis (CF) – a condition thousands of people are diagnosed with each year.
Using X-ray velocimetry (XV), the multi-disciplinary collaboration of physicists, engineers, biomedical engineers and clinicians was able to measure the dynamics of airflow through the lungs during the course of a natural breathing cycle, and measures the presentations of CF lung disease.
The research team, led by Dr Freda Werdiger from Monash University’s Department of Mechanical and Aerospace Engineering, was able to pinpoint the exact locations of abnormal airflow within lungs with CF-like disease, and better quantify the level of disease present.
The successful trial opens up avenues for a range of respiratory diseases to be diagnosed, treated and managed earlier than current technology allows and at a lower radiation dose than current CT scanning.
The study was published in the prestigious journal Scientific Reports.
“In this study we present two developments in XV analysis. Firstly, we show the ability of laboratory-based XV to detect the patchy nature of CF-like disease in affected mice. Secondly, we present a technique for numerical quantification of that disease, which can delineate between two major modes of disease symptoms,” Dr Werdiger said.
“This analytical model provides a simple, easy-to-interpret approach, and one capable of being readily applied to large quantities of data generated in XV imaging. Together these advances show the power of XV for assessing local airflow changes.
“We propose that XV should be considered as a novel lung function measurement tool for lung therapeutics development in small animal models, for CF and for other muco-obstructive diseases.”
Cystic fibrosis is a progressive, chronic and debilitating genetic disease caused by mutations in the CF Transmembrane-conductance Regulator gene. Unrelenting CF airway disease begins early in infancy and produces a steady deterioration in quality of life, ultimately leading to premature death.
Effective lung health assessment tools must capture the patchy nature of muco-obstructive lung diseases such as CF, which is important during the early stages of the disease when local treatments could be applied to prevent disease progression.
But, current lung function assessment methods have many limitations, including the inability to accurately localise the origin of globally-measured changes in lung health.
Assessments of overall lung health in humans and animals are made using lung function tests that screen for abnormalities by measuring the flow of gas in the airways.
Spirometry is the most common tool for assessing lung function, however it measures global airflow at the mouth.
Despite the availability of techniques that assess either lung function or lung structure, none of these are able to simultaneously quantify function and identify the origin of those functional changes. Abnormal lung motion during breathing has been demonstrated to be an indicator of disease.
X-ray velocimetry provides non-invasive, high-definition and sensitive real-time images of airflow through the lungs in live organisms.
The technology was designed and commercialised by Australian-based med-tech company 4DMedical, led by CEO and former Monash University researcher Professor Andreas Fouras. The technology has since been upscaled for clinical use, and in the USA was recently given FDA approval for all respiratory indications in adults.
“To effectively diagnose, monitor and treat respiratory disease clinicians should be able to accurately assess the spatial distribution of airflow across the fine structure of the lung. This capability would enable any decline or improvement in health to be located and measured, allowing improved treatment options to be designed,” Dr Werdiger said.
“The success of XV lies in its ability to draw reliable and meaningful quantitative measures, and this study shows how this can be accomplished.
“In the future, these techniques can be expected to be applied to the numerical characterisation of CF lung disease in larger cohorts and other CF animal models.
“These methods allow analyses to be applied in a straightforward fashion and with minimal manual processing, to enable ongoing study and development of the treatment of CF and other respiratory diseases.”
###
Collaboration on this project was undertaken with respiratory physicians at the Women’s and Children’s Hospital in Adelaide and the University of Adelaide. Gandel Philanthropy provided funding to assist in Dr Werdiger’s investigation.
For a full list of research contributors and to download a copy of the paper, please visit https:/
MEDIA ENQUIRIES
Monash University
T: +61 3 9903 4840 E: media@monash.edu
TDnews