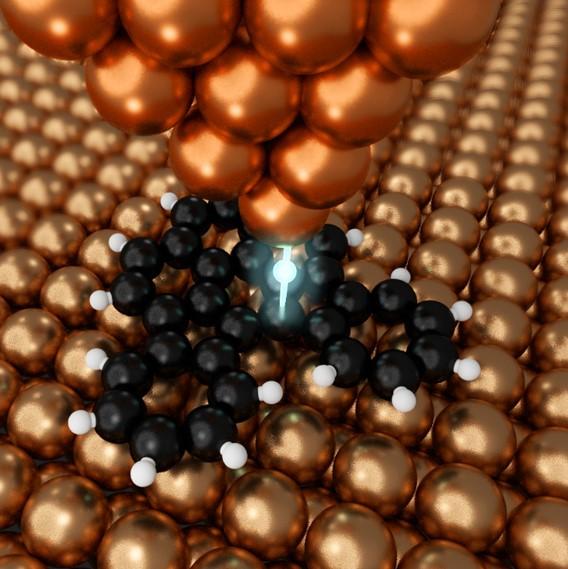
Photo: The copper probe can manipulate matter at the atomic scale.
view more
Credit Image: © 2020 Shiotari et al.
Nanographene is a material that is anticipated to radically improve solar cells, fuel cells, LEDs and more. Typically the synthesis of this material has been imprecise and difficult to control. For the first time, researchers have discovered a simple way to gain precise control over the fabrication of nanographene. In doing so, they have shed light on the previously unclear chemical processes involved in nanographene production.
You have probably heard of graphene, one-atom-thick sheets of carbon molecules, that are supposed to revolutionize technology. Units of graphene are known as nanographene; these are tailored to specific functions and as such their fabrication process is more complicated than that of generic graphene. Nanographene is made by selectively removing hydrogen atoms from organic molecules of carbon and hydrogen, a process called dehydrogenation.
“Dehydrogenation takes place on a metal surface such as that of silver, gold or copper, which acts as a catalyst, a material that enables or speeds up a reaction,” said Assistant Professor Akitoshi Shiotari from the Department of Advanced Materials Science. “However, this surface is large relative to the target organic molecules. This contributes to the difficulty in crafting specific nanographene formations. We needed a better understanding of the catalytic process and a more precise way to control it.”
Shiotari and his team, through exploring various ways to perform nanographene synthesis, came up with a method that offers the precise control necessary and is also very efficient. They used a specialized kind of microscope called an atomic force microscope (AFM), which measures details of molecules with a nanoscopic needlelike probe. This probe can be used not only to detect certain characteristics of individual atoms, but also to manipulate them.
“We discovered that the metal probe of the AFM could break carbon-hydrogen bonds in organic molecules,” said Shiotari. “It could do so very precisely given its tip is so minute, and it could break bonds without the need for thermal energy. This means we can now fabricate nanographene components in a more controlled way than ever before.”
To verify what they were seeing, the team repeated the process with a variety of organic compounds, in particular two molecules with very different structures called benzonoids and nonbenzonoids. This demonstrates the AFM probe in question is able to pull hydrogen atoms from different kinds of materials. Such a detail is important if this method is to be scaled up into a commercial means of production.
“I envisage this technique could be the ultimate way to create functional nanomolecules from the bottom up,” said Shiotari. “We can use an AFM to apply other stimuli to target molecules, such as injecting electrons, electronic fields or repulsive forces. It is thrilling to be able to see, control and manipulate structures on such an incredibly miniscule scale.”
###
Journal article
Akitoshi Shiotari, Ikutaro Hamada, Takahiro Nakae, Shigeki Mori, Tetsuo Okujima, Hidemitsu Uno, Hiroshi Sakaguchi, Yuji Hamamoto, Yoshitada Morikawa, and Yoshiaki Sugimoto. Manipulable Metal Catalyst for Nanographene Synthesis. Nano Letters.
DOI: 10.1021/acs.nanolett.0c03510. https:/
Funding
This work was supported by JSPS KAKENHI Grant Nos. JP16H00959, JP25110003, JP16H00967, JP15H06127, JP18H01807, JP18H03859, and JP18H05519. Akitoshi Shiotari acknowledges the support of ATI Research Grants 2017, Sumitomo Foundation, and Shimadzu Science Foundation. Yoshiaki Sugimoto acknowledges the support of Toray Science Foundation.
Useful links
Department of Advanced Materials Science
https:/
Graduate School of Frontier Sciences
https:/
Research contact
Assistant Professor Akitoshi Shiotari
Department of Advanced Materials Science, The University of Tokyo,
5-1-5 Kashiwanoha, Kashiwa-shi, Chiba 277-8561, JAPAN
Email: shiotari@k.u-tokyo.ac.jp
Press Contact
Mr. Rohan Mehra
Division for Strategic Public Relations, The University of Tokyo
7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8654, JAPAN
Tel: +81-(0)80-9707-8450
Email: press-releases.adm@gs.mail.u-tokyo.ac.jp
About the University of Tokyo
The University of Tokyo is Japan’s leading university and one of the world’s top research universities. The vast research output of some 6,000 researchers is published in the world’s top journals across the arts and sciences. Our vibrant student body of around 15,000 undergraduate and 15,000 graduate students includes over 4,000 international students. Find out more at http://www.
TDnews (tunisiesoir.com)














