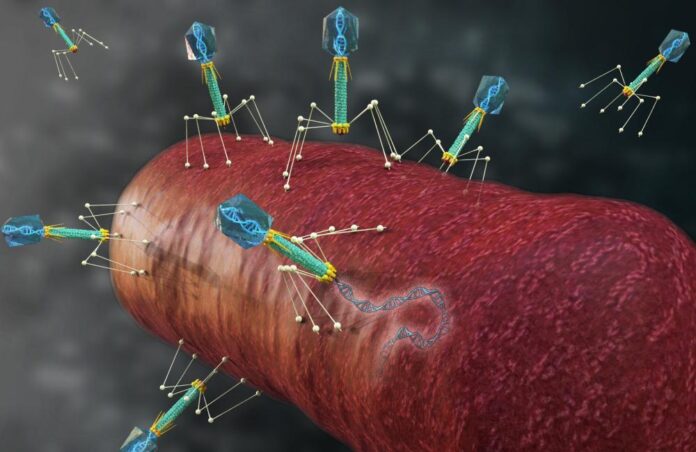
In the microscopic battlefield inside bacterial cells, a silent war is waging. Viruses known as phages—the natural predators of bacteria—invade, hijack, and multiply, bursting their hosts open in a relentless cycle. While harmless to humans, these viral invaders could hold the key to a future without antibiotics—if scientists can learn to control them.
At the Helmholtz Institute for RNA-based Infection Research (HIRI) in Würzburg, researchers led by Jörg Vogel have taken a major step toward that goal. Using an innovative RNA tool called antisense oligomers (ASOs), they have for the first time been able to selectively disrupt phage reproduction, essentially “hacking” the viral life cycle inside bacteria. The results, published in Nature, promise to transform both our understanding of phages and the therapeutic approaches that rely on them.
“Phages are like stealth guardians of our health,” says Vogel. “They decimate pathogenic bacteria as a side effect, but to harness their full potential, especially against antibiotic-resistant infections, we need to understand them at the molecular level.”
Traditional methods have struggled because phages cloak their genetic material from bacterial defenses and from researchers’ tools alike. The Würzburg team overcame this by introducing ASOs, which specifically block the synthesis of key phage proteins during replication. “We were able to pinpoint crucial proteins that drive phage propagation,” explains Milan Gerovac, first author of the study. “It’s like flipping switches inside the virus’s machinery.”
The researchers focused on ΦKZ, a jumbo phage capable of tackling Pseudomonas aeruginosa, a notorious hospital-acquired pathogen. By systematically silencing dozens of phage genes, they revealed previously unknown molecular players essential to viral reproduction—insights that could guide the design of next-generation phage therapies.
Beyond ΦKZ, the approach proved versatile across multiple phage-bacteria systems, offering a blueprint for understanding the vast, largely unexplored world of phage-host interactions. ASO technology, sometimes called programmable antibiotics or “asobiotics”, not only blocks bacterial protein synthesis but now shows promise in controlling the viruses that infect bacteria themselves.
“This is a breakthrough in molecular microbiology,” Vogel notes. “By manipulating phages at the genetic level, we can open entirely new avenues for treating infections and combating antibiotic resistance.”
As hospitals worldwide grapple with superbugs, the Würzburg study highlights a revolutionary possibility: rather than relying solely on traditional drugs, scientists could one day deploy phages—guided by RNA tools—to precisely target and eliminate deadly bacteria. In this microscopic war, human ingenuity may finally tip the balance.